- Home
- »
- Medical Devices
- »
-
U.S. Cell And Gene Therapy Clinical Trial Services Market Report 2030GVR Report cover
![U.S. Cell And Gene Therapy Clinical Trial Services Market Size, Share & Trends Report]()
U.S. Cell And Gene Therapy Clinical Trial Services Market Size, Share & Trends Analysis Report By Service (Site Identification, Patient Recruitment), By Phase (Phase I, Phase II), By Therapeutic Areas, By Therapy Type, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-4-68040-150-4
- Number of Pages: 183
- Format: Electronic (PDF)
- Historical Range: 2018 - 2022
- Industry: Healthcare
Market Size & Trends
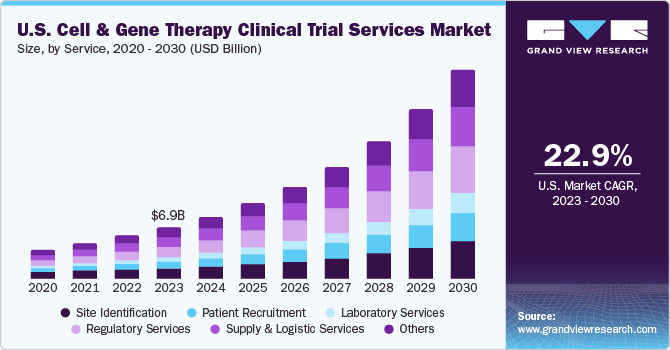
The U.S. cell and gene therapy clinical trial services market size was estimated at USD 6.87 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 22.9% from 2024 to 2030. The growing prevalence of cancer, the expanding cell and gene therapy (CGT) pipeline, and the rising number of biotechnology & pharmaceutical companies outsourcing their clinical trial services are expected to drive market growth during the forecast period. In addition, U.S. cell & gene therapy clinical trial services are backed by increasing investments and R&D funding, growing adoption of novel technologies across clinical research & supply chains, and the surge in mergers & acquisitions activities in the cell and gene therapy industry.
The COVID-19 pandemic has impacted the U.S. cell & gene therapy clinical trial services industry due to a slowdown of overall clinical trial activity in the early stages of the pandemic. The pandemic has led to massive disruption in supply chain management, which became the primary factor restraining the market growth. One of the main factors responsible is the general lack of demand forecast and feasibility planning. Logistics and transportation became primary concerns due to lockdowns and restrictions in the early stages of the pandemic. Clinical trials were delayed and temporarily suspended due to concerns regarding the safety of patients enrolled in trials and staff safety. As the pandemic stretched, with delays continuing for months, clinical trials that were in progress were disrupted as any inventory redundancies built into their supply chains eroded. Constant changes in regulatory policies related to clinical trial supplies adversely impacted the market.
However, the industry soon overcame the obstacles led by the outbreak of the COVID-19 pandemic, as the pandemic has accelerated certain new models in the clinical trial supplies industry to ease the crisis and cope with changing trends. Virtual trials gradually started gaining popularity and are expected to grow considerably in the coming years, which has significantly helped the industry to rebound revenues. Some of the major advantages of virtual trials are as follows:
-
They offer logistical convenience to patients. Various tools, such as wearable smart devices like smartphones, patches, apps, or other monitoring devices, help collect data remotely, reducing or eliminating the need for site visits.
-
The data can be captured virtually at any given time. This will shorten the processing time for sponsors and clinicians and allow patients to access their data securely and easily.
-
The virtual trials allow more diverse participants to be recruited. Clinical trials generally require a geographically dispersed diverse patient pool, which often becomes a challenge for contact-based trials. However, virtual trials can engage a diverse patient pool that can be recruited from anywhere, contributing to the market's resurgence after 2020.
Service Insights
The regulatory services segment held the largest revenue share of 21.8% in 2023. The cell and gene therapy industry's regulatory services provide guidance for enhancing the value of clinical trials throughout the product journey. This has led to a high demand for clinical trial services in the U.S., rapidly growing the segment. In March 2022, the FDA released draft guidance documents to support sponsors developing CGT products. The first draft is Human Gene Therapy Products Incorporating Human Genome Editing, which provides recommendations on the information to include in IND filings for the therapies, including safety, study design, and manufacturing. The second draft guidance is Considerations for Chimeric Antigen Receptor T Cell Products Development. Hence, such initiatives to support the development of CGT products support the market growth, simultaneously augmenting the demand for regulatory services.
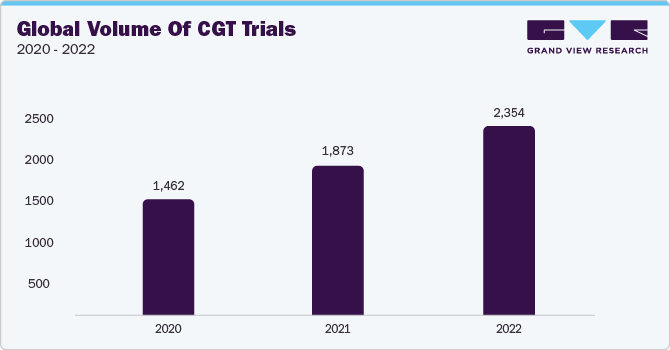
The supply and logistic services segment is anticipated to register a considerable CAGR of 23.3% over the forecast period. The segment has been further sub-categorized to include specific supply & logistic services, including logistics & distribution, storage & retention, packaging, labeling and blinding, manufacturing, comparator sourcing, and others. The segment is anticipated to witness a surge in demand owing to the growing number of clinical trials in the CGT space that require exclusive logistics and storage services for extremely delicate CGT materials. For instance, by evaluating several authentic sources such as the Alliance for Regenerative Medicine (ARM), U.S. FDA, Clinicaltrials.gov, and a few others, it can be inferred that as of 2022, the worldwide number of clinical trials for cell and gene therapy stands at around 2,354, exhibiting substantial growth in the past few years, as depicted below:
Phase Insights
The phase II segment held the largest revenue share of 48.4% in 2023. This is due to increasing generic product launches pertaining to cancer treatment. The expanding pipeline of cell and gene therapy candidates necessitates more Phase II trials to assess their safety and efficacy in diverse patient populations. Phase II clinical trials have emerging projects, and this trend is expected to continue owing to increasing investments in R&D by industry and nonindustrial sponsors. A growing number of industry-sponsored and non-industry-sponsored clinical trials in phase II, the complexity associated with phase II clinical trials, and the globalization of clinical trials are expected to boost the growth of the market, supporting the growth of the phase II segment.
On the other hand, the phase I segment is anticipated to register the fastest CAGR of 23.2% during the forecast period. Phase I study assesses the safety of a device or drug and involves evaluation of the tolerability and pharmacokinetics of molecules. It determines the effect of a device or drug on humans, including how it is absorbed, metabolized, and emitted. It also examines the side effects of the drug in case of increased dosage levels. The segment is anticipated to witness a lucrative growth rate across the analysis period owing to the rise in the clinical pipeline of CGT therapeutics. For instance, as per the U.S. FDA, around 13 new cell and gene therapies could secure approval in the U.S. and Europe by the end of 2023. Moreover, the U.S. FDA also stated that around 10 to 20 new cell and gene therapies will be approved by 2025.
Therapeutic Areas Insights
The oncology segment held the largest revenue share of 56.4% in 2023. This is due to increasing generic product launches pertaining to cancer treatment. Oncology is one of the most explored areas in terms of CGT product development. This is due to the increasing number of cancer cases across the U.S. and primarily the surge in oncology-related rare diseases. To curb the cancer burden, several pharmaceutical and biopharmaceutical companies are focusing on developing novel CGT therapeutics, thus supporting segmental growth. For instance, as per the American Society of Gene & Cell Therapy (ASGCT), in 2021, 75 new clinical trials have commenced in the field of CGT, with the top 10 diseases chosen for commencing trials all belonging to the field of oncology, with the leading three being related to blood cancers including nonHodgkin’s lymphoma (NHL), multiple myeloma, acute lymphocytic leukemia (ALL).
The central nervous system (CNS) disorders segment is anticipated to register the fastest CAGR of 24.7% during the forecast period. This is due to the rising prevalence of neurological diseases across the globe, including the U.S. Alzheimer’s disease, degenerative diseases, Huntington’s disease, Parkinson’s disease, expressive aphasia, and multiple sclerosis are some of the most common neurological diseases affecting people worldwide. Clinical trials & regulatory guidelines are boosting the entry of new products into the market. These drugs are monitored for dosage, formulation, efficacy, and aftereffects. For instance, in June 2022, the U.S. FDA nominated CNS Pharmaceuticals’ Berubicin, an orphan drug for treating malignant gliomas, a brain/CNS tumor. The major drivers of cell and gene therapy for CNS diseases are newer gene-editing tools, the introduction of safe & effective vectors, and huge unmet medical needs.
Therapy Type Insights
The cell therapy segment held the largest revenue share of 40.8% in 2023. This is due to increasing generic product launches pertaining to cancer treatment. Cell therapy services are vital in advancing regenerative medicine & personalized medicine. Demand for the therapy is rising across various diseases, including cancer, autoimmune conditions, neurological diseases, and more. Different specialized facilities, research institutions, and healthcare professionals with experience in cell-based therapies frequently provide cell therapy services. Cell therapy comprises various services, including logistics & distribution, to ensure that the therapy's components are safely kept, transported, and supplied to clinical sites. Along with a growing cell therapy pipeline, the increasing prevalence of diseases and novel innovations are expected to propel market expansion over the forecast period.
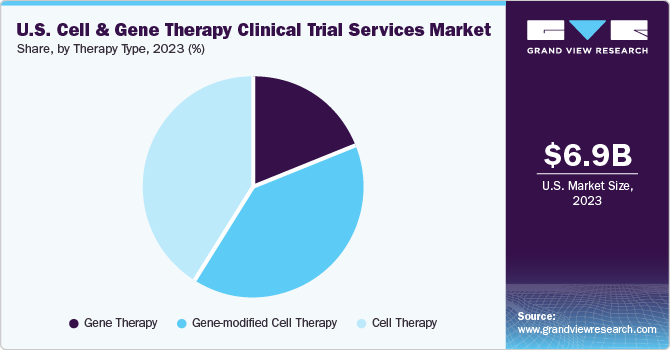
The gene-modified cell therapy segment is expected to register the fastest CAGR of 23.6% during the forecast period. In gene-modified cell therapy, specific cells are modified genetically outside the body to cure the disease. These therapies ideally modify cells, which are then returned to the patient to treat their disease. The segment has been further sub-categorized to include CAR T-cell therapies, TCR-T cell therapy, CAR-NK cell therapy, and others. Chimeric Antigen Receptor T-cell therapy is a gene-modified cell therapy used for blood cancers such as leukemia & lymphoma. Gene-modified stem cell therapy involves modifying stem cells, which have the potential to develop into various cell types, to treat conditions like inherited genetic disorders, blood disorders, and neurodegenerative diseases. Ex vivo gene therapy cells are removed from the patient, modified outside the body, and reintroduced. The increasing demand for gene-modified cell therapy creates a rising demand for clinical trial services supporting medical innovation.
Key Companies & Market Share Insights
The key players focus on expanding their facilities, product/service launches, collaborations, partnerships, and mergers and acquisitions. These are the key strategic initiatives influencing the industry dynamics. For instance, in January 2022, AmerisourceBergen and TrakCel launched the integrated platform to enhance access to prescribed cell & gene therapies, delivering complete visibility during the treatment. The two platforms are TrakCel’s advanced therapy orchestration platform, OCELLOS, and Fusion, a customer relationship management & patient support platform. Some prominent players in the U.S. cell and gene therapy clinical trial services market (Amalgamation of service providers by sponsor companies and logistic providers) are:
Key Cell And Gene Therapy (CGT) Clinical Trial Sponsors Companies:
- Novartis AG
- Takeda Pharmaceutical Company Limited.
- Gilead Sciences, Inc
- Amgen Inc.
- bluebird bio, Inc.
- Regeneron Pharmaceuticals Inc.
- CRISPR Therapeutic
- Pfizer
Key Cell And Gene Therapy (CGT) Supply & Logistic Providers Companies:
- Biocair
- Modality Solutions LLC
- BioLife Solutions, Inc.
- Marken
- Yourway
- Almac Group
- Catalent, Inc
- Quick International Courier (QuickStat (Kuehne + Nagel Company)
U.S. Cell And Gene Therapy Clinical Trial Services Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 8.30 billion
Revenue forecast in 2030
USD 28.58 billion
Growth rate
CAGR of 22.9% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion, and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, and trends
Segments covered
Service, phase, therapeutic areas, therapy type
Country scope
U.S.
Key companies profiled
Novartis AG; Takeda Pharmaceutical Company Limited.; Gilead Sciences, Inc.; Amgen Inc.; bluebird bio, Inc.; Regeneron Pharmaceuticals Inc.; CRISPR Therapeutics; Pfizer; Biocair; Modality Solutions LLC; BioLife Solutions, Inc.; Marken; Yourway; Almac Group; Catalent, Inc; Quick International Courier (QuickStat (Kuehne + Nagel Company)
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
U.S. Cell And Gene Therapy Clinical Trial Services Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis on the latest industry trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the U.S. cell and gene therapy clinical trial services market report on the basis of service, phase, therapeutic areas, and therapy type.
-
Service Outlook (Revenue, USD Million, 2018 - 2030)
-
Site Identification
-
Patient Recruitment
-
Laboratory Services
-
Regulatory Services
-
Supply And Logistic Services
-
Logistics & Distribution
-
Storage & Retention
-
Packaging, Labeling, And Blinding
-
Manufacturing
-
Comparator Sourcing
-
Regulatory and customs support
-
Other Services
-
-
Others
-
-
Phase Outlook (Revenue, USD Million, 2018 - 2030)
-
Phase I
-
Phase II
-
Phase III
-
Phase IV
-
-
Therapeutic Areas Outlook (Revenue, USD Million, 2018 - 2030)
-
Oncology
-
Central Nervous System (CNS) Disorders
-
Cardiovascular Diseases
-
Infectious Diseases
-
Musculoskeletal
-
Others
-
-
Therapy Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Gene Therapy
-
Gene-modified Cell Therapy
-
CAR T-cell therapies
-
CAR-NK Cell Therapy
-
TCR-T Cell Therapy
-
Others
-
-
Cell Therapy
-
Frequently Asked Questions About This Report
b. The U.S. cell and gene therapy clinical trial services market size was estimated at USD 6.87 billion in 2023 and is expected to reach USD 8.30 billion in 2024
b. The U.S. cell and gene therapy clinical trial services market is expected to register a double-digit growth rate of 22.9% from 2024 to 2030. The collective pipeline for gene, cell, and RNA therapies, ranging from preclinical stages to pre-registration, is expected to augment the market growth.
b. Regulatory Services dominated the U.S. cell and gene therapy clinical trial services market with the highest share of 21.8% in 2022. Regulatory services provide guidance for enhancing the value of clinical trials throughout the product journey, leading to high demand for U.S. clinical trial services.
b. Some key players operating in the U.S. cell and gene therapy clinical trial services market are Biocair; MODALITY SOLUTIONS; BioLife Solutions, Inc; Marken; Yourway; Almac Group; Catalent, Inc; Be The Match BioTherapies; AmerisourceBergen Corporation; Thermo Fisher Scientific (Patheon); Biostor Systems Inc; Arvato Supply Chain Solutions SE; Quick International Courier [QuickStat (a Kuehne + Nagel company)]; and Trakcel.
b. Some of the key factors driving the U.S. cell and gene therapy clinical trial services market are increasing investments in R&D, expanding biologics pipeline, growing adoption of novel technologies across clinical research & supply chains, and the rising number of global CROs. Moreover, stringent regulations pertaining to clinical trial design and logistics have further increased market demand.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![ESOMAR Certified Member]()
![Great Place to Work Certified]()
ESOMAR & Great Work to Place Certified
![ISO 9001:2015 & 27001:2022 Certified]()
ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."







