- Home
- »
- Clinical Diagnostics
- »
-
Prostate Cancer Diagnostics Market Size, Share Report 2030GVR Report cover
![Prostate Cancer Diagnostics Market By Test Type (Preliminary, Confirmatory), By Type (Adenocarcinoma, Interstitial cell carcinoma), By End Use (Hospitals, Outpatient Facilities), By Region And Segment Forecasts, 2023 - 2030Report]()
Prostate Cancer Diagnostics Market By Test Type (Preliminary, Confirmatory), By Type (Adenocarcinoma, Interstitial cell carcinoma), By End Use (Hospitals, Outpatient Facilities), By Region And Segment Forecasts, 2023 - 2030
- Report ID: GVR-2-68038-548-9
- Number of Pages: 95
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
The global prostate cancer diagnostics market size was valued at USD 8.1 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.23% from 2023 to 2030. The increasing prevalence of prostate cancer globally is one of the major factors propelling demand for diagnostic products. According to the National Cancer Institute, in 2019, over 174,650 new cases of prostate cancer were diagnosed in the U.S. Additionally, data from the same source also suggests that over 31,620 deaths occurred in 2019 due to prostate cancer.
Technological advancements in this market are further expected to fuel growth during the forecast period. According to the National Cancer Institute, researchers are currently testing the use of artificial intelligence (AI) to diagnose prostate cancer. AI tools are used to identify suspicious areas identified during MRI scans that may require biopsies to confirm the presence of malignant cells. AI tools tested to improve biopsy sample analysis help in providing efficient and accurate results.
Supportive government initiatives towards developing new technologies to enhance diagnosis is further expected to boost market growth. For instance, the National Cancer Institute initiated Prostate Specialized Programs of Research Excellence (SPORE) which is designed to convert basic scientific findings into clinical settings. They support the development of new technologies and studies to gain a better understanding of monitoring, prevention, diagnosis, and treatment.
The rising number of new market entrants is another factor anticipated to fuel market growth during the forecast period. For instance, in July 2018, Gregor Diagnostics invested USD 9,00,000 to support its efforts to develop a novel at-home screening diagnosis test. This device is anticipated to provide highly accurate test results as compared to common PSA tests. Thus, a rise in investment by new market entrants is expected to rapidly increase the demand for diagnostic products.
End Use Insights
Based on end use, the market is segmented into hospitals, outpatient facilities, home care, and research & manufacturing. In 2022, the outpatient facilities segment dominated the market. Outpatient Facilities have revolutionized and developed more comfortable consulting and treatment systems with the acquisition and experience of the licensed physician. The preference of patience coupled with technological advancements is expected to surge the market growth.
In addition, the level of comfort and ease of consulting physicians have elevated the segment’s growth. Moreover, increased patient footfall in outpatient facilities in the pre-diagnostic phase is further anticipated to impact the market size. The home care segment is expected to register the highest CAGR of 8.00% during the forecast period owing to the rising geriatric population.
Type Insights
Based on type, the market is segmented into adenocarcinoma, interstitial cell carcinoma, and others. In 2022, the adenocarcinoma segment dominated the market with 75.11% share. Adenocarcinoma is a cancer of the glandular tissue, and it forms lines in multiple internal organs. Most adenocarcinoma ejects substances such as mucus, digestive juices, and other fluids. These affect the breast, lungs, esophagus, colon, rectum, prostate, and uterus. Cancer found in the prostate gland is typically prostate adenocarcinoma.
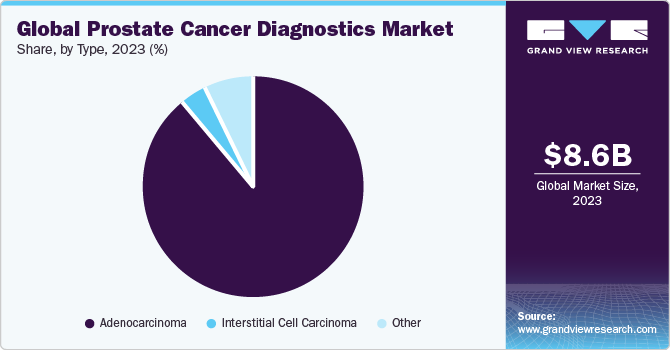
According to an article published by the Cancer Treatment Center of America 99.0%of all prostate cancers are Prostate Adenocarcinoma. Hence, the rising number of prostate cancer patients is anticipated to fuel the Adenocarcinoma segment’s growth.
Test Type Insights
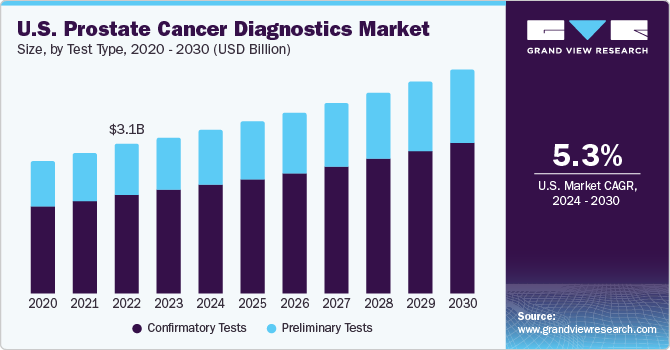
Based on test type, the market is analyzed for preliminary and confirmatory tests. Confirmatory tests accounted for the largest market share in 2022 with 66.31%. Confirmatory tests include the PCA3 test, Transrectal Ultrasound (TRUS), and biopsy of the affected site. Post preliminary testing, if results of PSA and DRE are abnormal, patients are required to undergo confirmatory tests to diagnose the disease. With rising disease prevalence globally, there has been an increase in the usage of confirmatory tests, which fuels the segment’s growth. The same segment is expected to register the highest CAGR during the forecast period.
The rising incidence of prostate cancer is further anticipated to surge the segment’s growth. According to the National Cancer Institute, an estimated number of 3,343,976 men in the U.S. are currently living with prostate cancer.
Other preliminary tests include Digital Rectal Exam (DRE) and biomarker tests. DRE tests are used to detect hard, lumpy as well as abnormal growth of the prostate gland. These tests, conducted by doctors, depend on their skills and abilities, and the results obtained may not be accurate and, in most instances, require further validation by conducting PSA and biopsy tests.
Regional Insights
The North American region held the largest market with a 45.12% share owing to the rising prevalence of prostate cancer and favorable government initiatives to enhance diagnosis. For instance, the National Cancer Institute initiated several programs to enhance research efforts for early disease detection and treatment. Some of their programs include a biomarkers research group that promotes research on biomarkers. The institute also manages the Early Detection Research Network (EDRN), a network of the National Cancer Institute that collaborates with other such organizations to discover as well as validate early detection of biomarkers. The rise in disease prevalence in the North American region is expected to further fuel the market growth during the forecast period. According to the American Institute for Cancer Research, prostate cancer is the second most occurring cancer in males in the U.S. Thus, the rising incidence of this disease is anticipated to fuel market growth in the near future.
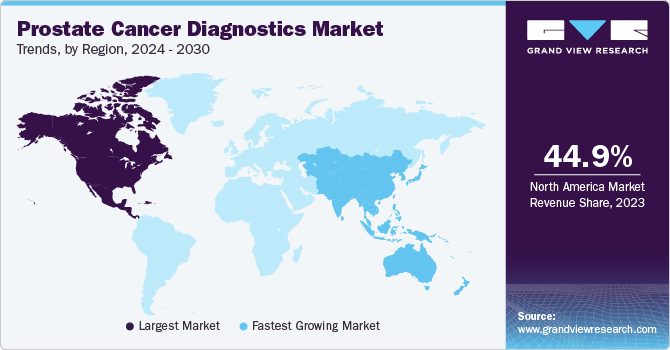
The Asia Pacific region is expected to witness the fastest growth due to a rise in disease prevalence. According to the National Institute of Health, disease epidemiology in Asian countries is increasing due to lifestyle changes such as a rise in the consumption of Westernized diets and other lifestyle changes. Increasing awareness regarding prostate cancer and government-initiated prevention programs is anticipated to drive the prostate cancer diagnostic market.
Key Companies & Market Share Insights
The launch of new products by leading market players is anticipated to impact market growth positively. Strategic initiatives, such as mergers and acquisitions, the development of technologically advanced devices, and a rising number of product launches are expected to boost the adoption of prostate cancer diagnostic products during the forecast period. New product launches are anticipated to impact market growth positively.
For instance, in 2017, Roche launched a new cancer biomarker, the rabbit monoclonal primary antibody anti-p504s (SP116) for disease diagnosis. The biomarker is used for detecting α-methylacyl-CoA racemase in tissue sections, enabling easy identification of morphologically different samples such as cancerous, benign, and atypical from a single slide. In June 2018, MDxHealth announced an agreement with Philips for the rights to manufacture and commercialize Philips’ prognostic biomarker phosphodiesterase-4D7 as a prognostic test for the diagnosis. This agreement is intended to aid MDxHealth in preparing for the launch of InformMDx test commercially. In February 2018, Genomic Health Inc. launched the Oncotype DX AR-V7 Nucleus Detect liquid biopsy test in the U.S. Some of the prominent key players in the global prostate cancer diagnostics market include:
-
MDx Health,
-
Myriad Genetics, Inc.,
-
Abbott Laboratories,
-
F. Hoffman-La Roche AG,
-
Siemens Healthcare GmbH,
-
OPKO Health, Inc.
-
Genomic Health.
Prostate Cancer Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 8.56 billion
Revenue forecast in 2030
USD 13.16 billion
Growth rate
CAGR of 6.23% from 2023 to 2030
Base year for estimation
2022
Historic data
2018 - 2021
Forecast period
2023 - 2030
Report updated
September 2023
Quantitative units
Revenue in USD million/billion & CAGR from 2023 to 2030
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, and trends
Segments covered
Test type, type, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
MDx Health; Myriad Genetics, Inc.; Abbott Laboratories; F. Hoffman-La Roche AG; Siemens Healthcare GmbH; OPKO Health, Inc.; Genomic Health.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional, and segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Prostate Cancer Diagnostics Market Report Segmentation
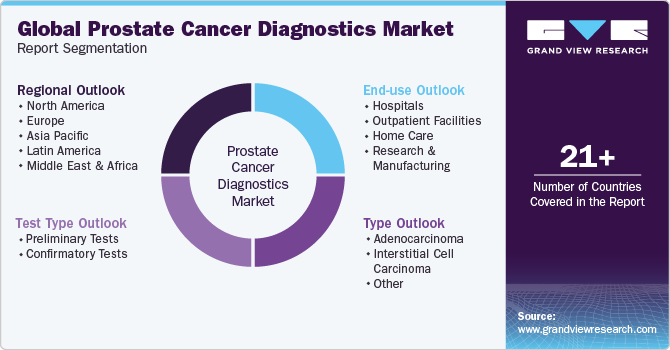
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global prostate cancer diagnostics market report based on test type, type, end-use, and region:
-
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Preliminary Tests
-
PSA Tests
-
Free PSA Test
-
Total PSA Test
-
-
Other Preliminary Tests
-
-
Confirmatory Tests
-
Pca3 Test
-
Trans-Rectal Ultrasound
-
Biopsy Test
-
-
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Adenocarcinoma
-
Interstitial Cell Carcinoma
-
Other
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Outpatient Facilities
-
Home Care
-
Research & Manufacturing
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global prostate cancer diagnostics market size was estimated at USD 8.1 billion in 2022 and is expected to reach USD 8.56 billion in 2023.
b. The global prostate cancer diagnostics market is expected to grow at a compound annual growth rate of 6.23% from 2023 to 2030 to reach USD 13.16 billion by 2030.
b. Preliminary tests dominated the prostate cancer diagnostics market with a share of 66.31% in 2022. This is attributable to the easy availability of PSA screening which has facilitated early diagnosis and treatment, increasing survival rates, thereby driving growth.
b. Some key players operating in the prostate cancer diagnostics market include MDx Health; Myriad Genetics, Inc.; Abbott Laboratories; F. Hoffman-La Roche AG; Siemens Healthineers AG; OPKO Health, Inc.; and Genomic Health.
b. Key factors that are driving the market growth include increasing prevalence of prostate cancer, technological advancements in diagnostic tests, and the use of Artificial Intelligence (AI) to diagnose prostate cancer.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![ESOMAR Certified Member]()
![Great Place to Work Certified]()
ESOMAR & Great Work to Place Certified
![ISO 9001:2015 & 27001:2022 Certified]()
ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."

Important: Covid19 pandemic market impact
The most common concern for the governments of all Covid-19 hit nations is the excruciating need to screen for and test large numbers of patients for possible Sars-Cov-2 infection. As a result, most of them are facing major shortages in the supply for diagnostic kits to test for the virus. Diagnostics virology entities are under immense pressure to provide reliable testing kits, and there is a surge in demand for in-vitro or point-of-care testing capacities by labs across a large number of countries. The report will account for Covid19 as a key market contributor.






