- Home
- »
- Biotechnology
- »
-
Gene Therapy Market Size, Share & Growth Report, 2030GVR Report cover
![Gene Therapy Market Size, Share & Trends Report]()
Gene Therapy Market Size, Share & Trends Analysis Report By Indication (Acute Lymphoblastic Leukemia, Large B-cell Lymphoma), By Vector Type (Lentivirus, Adenovirus), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-2-68038-179-5
- Number of Pages: 162
- Format: Electronic (PDF)
- Historical Range: 2018 - 2022
- Industry: Healthcare
Gene Therapy Market Size & Trends
The global gene therapy market size was estimated at USD 8.67 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 19.5% from 2024 to 2030. The growth of the market is attributed to many factors such as expanding area of advanced therapies along with gene delivery technologies and progressive competition among key players focused on commercialization of their therapies. The biotechnology companies are investing in acquisitions, mergers/collaborations, and deals as a key strategy to increase in-house expertise and strengthen the product pipelines.
The COVID-19 outbreak has negatively impacted the market growth. This sector has experienced severe disruption due to COVID-19, which has historically presented significant challenges in the supply of materials, manufacturing, and logistics operations. For instance, companies had lengthy delivery times for specific components and later discovered that it was short on clinical trial supplies when a partner contract manufacturing company was compelled to shut down.
The robust pipeline is expected to boost the market growth over the forecast period. Researchers are working to make gene therapy available at clinics. Various universities and institutes exhibit a broad portfolio of products in the pipeline which is expected to boost revenue generation over the forecast period. Clinical trials for gene therapy increased significantly from 2017 to 2018, after the FDA approved first gene therapy. According to the American Society of Gene & Cell Therapy (ASGCT), around 1,986 products, including CAR T-cell therapies and other genetically modified cell therapies, are currently under development.
Moreover, improving regulatory support creates growth opportunities for the market over the forecast period. Several positive changes have been made by many international regulatory organizations to promote therapies. Support for CAR-T technology from the FDA is one of the examples. In phase II and III studies, in particular, regulators are allowing flexibility in the usual hierarchy of how clinical trials are conducted. Moreover, FDA expects 10 to 20 new therapies to be approved annually by 2025.
Furthermore, an increase in funding and investments in this sector is expected to provide lucrative growth opportunities to market players. Several biopharma companies are investing in this sector for novel product launches. For instance, in January 2022, Ori Biotech raised more than USD 100.0 million in Series B funding to introduce a novel cell & gene therapy developing platform. This funding allowed for a rapid transition from pre-commercialization to market launch.
Market Dynamics
Researchers are working to make gene therapy available at clinics. Although very few patients have so far received effective treatments, gene therapy holds high potential in revolutionizing disease treatment regimens by targeting the genes responsible for disease pathogenesis. Various universities and institutes are observed to exhibit a broad portfolio in the pipeline, which is expected to boost revenue generation in near future. Clinical trials for gene therapy have increased significantly from 2017 to 2018 after the FDA approved the first therapy. This first approval propelled the confidence of entities in the gene therapy field. The following charts show the number of products under clinical trials.
With rising demand for robust disease treatment therapies, companies are accelerating R&D efforts for effective therapies that target the cause of disease at a genomic level. According to clinicaltrials.gov, the number of cell & gene therapies across global pipeline programs (Phase I to Phase III trials) increased from 289 in 2018 to 327 in 2022. Furthermore, the U.S. FDA provides constant support for innovations in this arena via several policies concerning product manufacturing. For instance, in October 2021, the National Institutes of Health, the FDA, five nonprofit organizations, and 10 pharmaceutical companies entered into a partnership to ramp up the development of novel therapies for 30 million individuals in the U.S. who suffer from rare disorders.
Vector Type Insights
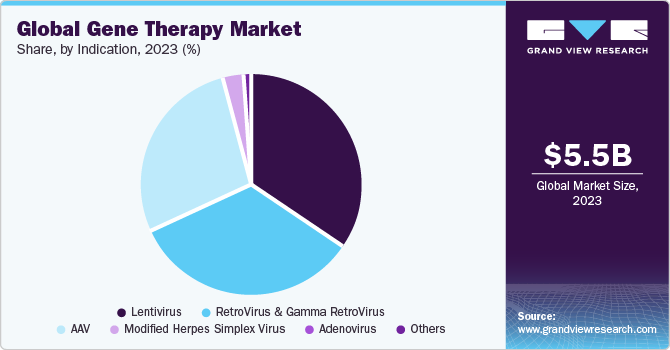
The AAV segment shows a significant revenue contribution of 22% in 2023. Several biopharma companies are offering their viral vector platform for the development of AAV-based gene therapy product. For instance, in September 2016, Lonza signed an exclusive agreement with Massachusetts Eye and Ear to support its novel Anc-AAV gene therapy platform for development and commercialization of next-generation gene therapies based on their AAV platform. Similarly, RegenxBio had made an agreement with companies AveXis & Biogen in March 2014 and May 2016, respectively, which would allow both companies to use RegenxBio’s AAV vector platform for development of gene therapy molecules. Furthermore, in May 2021, Biogen Inc. and Capsigen Inc. entered into a strategic research partnership to engineer novel AAV capsids that have the possibility to deliver transformative gene therapies, which can address the fundamental genetic causes of numerous neuromuscular and CNS disorders. In July 2021, the U.S. Department of Commerce’s National Institute of Standards and Technology (NIST), National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL), and United States Pharmacopeia (USP) announced a collaboration to evaluate analytical methods and develop standards for AAV. As part of this partnership, NIST and USP will be conducting an interlaboratory study in which several laboratories will measure these serious quality attributes, and their results will be linked and examined. This collaboration will support the development of new promising gene therapies that will significantly advance people’s lives.
Indication Insights
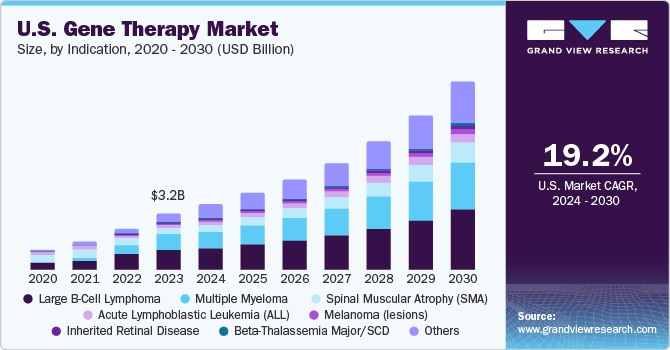
The spinal muscular atrophy (SMA) segment dominated the market in 2023 with a share of 46.8%. Although SMA is a rare disorder, it is one of the most common fatal inherited diseases of infancy. The development of Zolgensma (AVXS-101), has proven its effectiveness in treating SMA and altering the phenotype of the illness. The FDA approved Novartis' Zolgensma approval in May 2019, which is aimed at treating the underlying cause of SMA. As of now, Zolgensma is the only gene treatment in this field to have been approved. The approval of this gene therapy is evidence of the growing use of therapies to treat serious hereditary illnesses like SMA.
The Beta-Thalassemia Major/SCD segment is anticipated to register the fastest CAGR of 38.3% over the forecast period. Gene therapy for SCD and β-thalassemia is based on transplantation of gene-modified hematopoietic stem cells. Clinical and preclinical studies have shown the efficacy and safety of this therapeutic modality. However, several other factors, such as suboptimal gene expression levels & gene transfer efficiency, limited stem-cell dose and quality, and toxicity of myeloablative regimens are still hampering its efficacy. Despite these challenges, in June 2019, bluebird Bio’s Zynteglo (formerly LentiGlobin) received conditional approval in Europe for the treatment of β-thalassemia and is expected to receive U.S. FDA approval in August 2022. Moreover, the product has already received Orphan Drug status by the U.S. FDA for treatment of patients with sickle cell disease (SCD). Furthermore, in April 2021, Vertex Pharmaceuticals and CRISPR Therapeutics amended partnership for the development, production, and commercialization of CTX001 in sickle beta thalassemia and cell disease. These achievements in this segment are anticipated to significantly boost the adoption of the product in this segment.
Regional Insights
North America dominated the market in 2023 with the largest revenue share of 65.2% in 2023. This region is expected to become the largest routine manufacturer of gene therapy in terms of the number of approvals and revenue generated during the forecast period. Increasing investments in R&D from large and small companies in the development of ideal therapy drugs are anticipated to further boost the market.
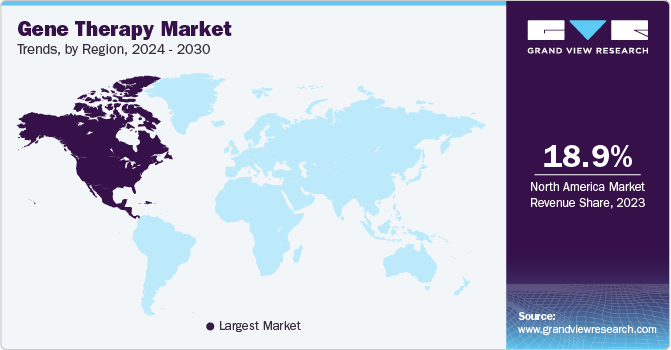
Furthermore, the increasing number of investments by the governments and the growing prevalence of targeted diseases are the factors fueling the market. According to the Spinal Muscular Atrophy Foundation, in 2020, around 10,000 to 25,000 children and adults in the U.S. were affected by spinal muscular atrophy, making it a fairly common disease among rare diseases.
Europe is estimated to be the fastest-growing regional segment from 2024 to 2030. This is attributed to its large population with unmet medical needs and increasing demand for novel technologies in the treatment of rare but increasingly prevalent diseases. Asia Pacific market for commercial application of genetic therapies is anticipated to witness significant growth in the forecast period, which can be attributed to the easy availability of resources, local presence of major companies, and increased investment, by the governments.
Key Companies & Market Share Insights
A rise in investment and funding for the advancements in this field is anticipated to provide growth opportunities to the companies. Furthermore, key players are engaged in strategic initiatives to boost their offerings in the market, which is expected to strengthen the market competition during the coming years. Some of the major initiatives taken by the company include:
-
For instance, in January 2022, 64x Bio, a U.S.-based biotech company, raised USD 55.0 million in funding to advance its gene therapy manufacturing platform. This initiative was expected to expand the company’s VectorSelect platform.
-
In April 2022, Pfizer, Inc. announced its plan to introduce its first U.S. site in the Phase 3 trials of investigational gene therapy for Patients with Duchenne Muscular Dystrophy. This initiative started after approval for restarting the global Phase 3 CIFFREO study from regulatory authorities in Canada, Taiwan, the UK, Spain, and Belgium.
-
In January 2023, Voyager Therapeutics and Neurocrine Biosciences entered into a strategic collaboration for commercialization & development of Voyager’s GBA1 program and other next-generation gene therapies for neurological diseases.
-
In January 2023, Spark Therapeutics and Neurochase established a strategic collaboration to develop Neurochase’s unique delivery technology for use with selected gene treatments for rare disorders in the CNS. In this agreement, Neurochase will contribute its extensive knowledge in direct drug delivery technology to Spark’s premier AAV platform.
Key Gene Therapy Companies:
- REGENXBIO, Inc.
- Oxford BioMedica plc
- Dimension Therapeutics, Inc.
- Bristol-Myers Squibb Company
- SANOFI
- Applied Genetic Technologies Corporation
- F. Hoffmann-La Roche Ltd
- Bluebird Bio, Inc.
- Novartis AG
- Taxus Cardium Pharmaceuticals Group, Inc. (Gene Biotherapeutics)
- UniQure N.V.
- Shire Plc
- Cellectis S.A.
- Sangamo Therapeutics, Inc
- Orchard Therapeutics
- Gilead Lifesciences, Inc.
- BENITEC BIOPHARMA
- Sibiono GeneTech Co., Ltd
- Shanghai Sunway Biotech Co., Ltd.
- Gensight Biologics S.A.
- Transgene
- Calimmune, Inc.
- Epeius Biotechnologies Corp.
- Astellas Pharma Inc.
- American Gene Technologies
- BioMarin Pharmaceuticals, Inc.
Gene Therapy Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 10.11 billion
Revenue forecast in 2030
USD 29.51 billion
Growth rate
CAGR of 19.5% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Report updated
November 2023
Quantitative units
Revenue in USD million/billion, CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, trends
Segments covered
Vector type; indication; region
Regional scope
North America; Europe; Asia Pacific; Rest of the World
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Japan; China; South Korea; Australia
Key companies profiled
REGENXBIO, Inc.; Oxford BioMedica plc; Dimension Therapeutics, Inc.; Bristol-Myers Squibb Company; SANOFI; Applied Genetic Technologies Corp; F. Hoffmann-La Roche Ltd.; Bluebird Bio, Inc.; Novartis AG; Taxus Cardium Pharmaceuticals Group, Inc. (Gene Biotherapeutics); UniQure N.V.; Shire Plc; Cellectis S.A.; Sangamo Therapeutics, Inc.; Orchard Therapeutics; Gilead Lifesciences, Inc.; Benitec Biopharma Ltd.; Sibiono GeneTech Co., Ltd.; Shanghai Sunway Biotech Co., Ltd.; Gensight Biologics S.A.; Transgene; Calimmune, Inc.; Epeius Biotechnologies Corp.; Astellas Pharma, Inc.; American Gene Technologies; BioMarin Pharmaceuticals, Inc.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Gene Therapy Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels in addition to providing an analysis of the latest industry trends in each of the subsegments from 2018 to 2030. For this study, Grand View Research has segmented the global gene therapy market report based on indication, vector type, and region:
-
Indication Outlook (Revenue, USD Million, 2018 - 2030)
-
Acute Lymphoblastic Leukemia (ALL)
-
Inherited Retinal Disease
-
Large B-cell Lymphoma
-
ADA-SCID
-
Melanoma (lesions)
-
Beta-Thalassemia Major/SCD
-
Head & Neck Squamous Cell Carcinoma
-
Peripheral arterial disease
-
Spinal Muscular Atrophy (SMA)
-
Others
-
-
Vector Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Lentivirus
-
AAV
-
RetroVirus & gamma RetroVirus
-
Modified Herpes Simplex Virus
-
Adenovirus
-
Non-viral Plasmid Vector
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
-
Asia Pacific
-
Japan
-
China
-
South Korea
-
Australia
-
-
Rest of the world
-
Frequently Asked Questions About This Report
b. The global gene therapy market size was estimated at USD 8.67 billion in 2023 and is expected to reach USD 10.12 billion in 2024.
b. The global gene therapy market is expected to grow at a compound annual growth rate of 19.5% from 2024 to 2030 to reach USD 29.51 billion by 2030.
b. North America dominated the gene therapy market with a share of 65.2% in 2023. This is attributable to rising healthcare awareness coupled with the growing demand for robust therapeutics to treat chronic illness.
b. Some key players operating in the gene therapy market include Novartis AG; Spark Therapeutics LLC; Bluebird Bio; Gilead Sciences Inc.; Celgene Corporation; Shire Plc; Voyager Therapeutics; Dimension Therapeutics; Chiesi Farmaceutici S.p.A; and Celgene Corporation.
b. Key factors that are driving the gene therapy market growth include recent approval of products such as Zolgensma and LentiGlobin which has accelerated investment in clinical trials of pipeline programs.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![ESOMAR Certified Member]()
![Great Place to Work Certified]()
ESOMAR & Great Work to Place Certified
![ISO 9001:2015 & 27001:2022 Certified]()
ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."

Important: Covid19 pandemic market impact
The outbreak of COVID-19 has negatively impacted market growth. This sector has experienced severe disruption as a result of COVID-19, which has historically presented significant challenges in the supply of materials, manufacturing, and logistics operations. The report will account for COVID-19 as a key market contributor.






